Abstract
Transplantation of genetically modified autologous hematopoietic stem and progenitor cells (HSPCs) holds a curative potential for subjects with inherited blood disorders. In recent years, transfer of a therapeutic gene to HSPCs has been successfully achieved using replication-incompetent integrating lentiviral vectors. More recently, advances have emerged to more precisely edit cellular genomes by specific correction of mutations or targeted gene addition at endogenous genomic loci. However, cellular processes triggered in HSPCs by the programmable nucleases utilized in these gene editing approaches may negatively impact their ability to reconstitute and maintain hematopoiesis long-term in recipient hosts. Granulocyte colony-stimulating factor (G-CSF) use after autologous HSPC transplantation is generally recommended to shorten the duration of severe neutropenia. However, little is known about the safety and efficacy of G-CSF use after transplantation of genetically modified autologous HSPCs. G-CSF is the principal cytokine regulating granulopoiesis, but also plays an important role in regulating hematopoietic stem cell (HSC) function (Schuttpelz, Leukemia 2014). Studies have suggested that G-CSF can exacerbate HSC damage caused by chemotherapeutic agents and irradiation by promoting differentiation at the expense of self-renewal and by inducing cellular senescence (van Os, Stem Cells 2000; Li, Cell Biosci 2015). Here, we asked whether G-CSF use after transplantation of gene edited HSPCs may negatively affect their long-term repopulating (LTR) and self-renewal capacities.
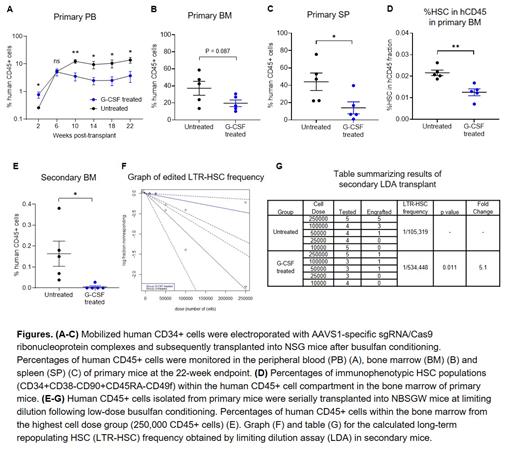
To assess the effect of G-CSF use post-transplant on HSPC repopulating function after gene editing, mobilized human CD34+ cells were stimulated for 2 days, electroporated with AAVS1-specific sgRNA/Cas9 ribonucleoprotein complexes, and subsequently transplanted into NSG mice following busulfan conditioning. We subcutaneously injected G-CSF (125 mcg/kg/day) or PBS from post-transplant day 1 to 14 and compared hematopoietic reconstitution between both groups. The use of G-CSF initially increased human CD45+ cells in peripheral blood (PB) at 2 weeks post-transplant by enhancing CD13+ myeloid cell proliferation from committed progenitors (Fig. A). However, starting at 10 weeks post-transplant when hematopoiesis begins to emerge from the most primitive HSPCs, administration of G-CSF resulted in a 3 to 4-fold reduction in PB human cell engraftment compared to untreated mice (Fig. A). Similarly, G-CSF treated mice had significantly lower bone marrow (BM) and splenic engraftment at the endpoint (22 weeks) analysis, with comparable editing efficiency and lineage composition detected within human CD45+ cells (Fig. B, C). Importantly, percentages of immunophenotypic HSCs were 2-fold lower within the BM of G-CSF treated mice relative to the untreated group (Fig. D). To determine whether the negative effect of G-CSF post-transplant is specific to CRISPR-Cas9 gene editing, similar experiments were conducted using unmanipulated CD34+ cells or CD34+ cells transduced with a lentivirus vector expressing GFP. Interestingly, we found no differences in engraftment levels or immunophenotypic HSC frequencies between G-CSF treated and untreated mice. To assess the self-renewal capacity and quantify the frequency of gene edited LTR-HSCs, human CD45+ cells obtained from the BM of primary mice were serially transplanted into secondary recipient (NBSGW) mice at limiting dilution and BM engraftment was analyzed at 20 weeks post-transplant (total period of engraftment was 42 weeks). Notably, the secondary mice in the untreated group showed significantly superior human CD45+ cell engraftment compared with those in G-CSF treated group at the highest dose tested (Fig. E). The extreme limiting dilution analysis indicated that the frequency of LTR-HSCs was 5.1-fold higher (p = 0.011) in the untreated group compared with G-CSF treated group (Fig. F, G). Considering total engraftment in primary mice and the frequency of edited LTR-HSCs in secondary mice, we estimated the frequency of edited LTR-HSCs was reduced by 10-fold with G-CSF administration post-transplant.
Collectively, our data suggest that G-CSF use post-transplant significantly reduces LTR and self-renewal capacities of CRISPR-Cas9 gene edited HSPCs. This understanding could have important clinical implications in HSPC gene therapy protocol.
No relevant conflicts of interest to declare.